Konbanwa!!!
Ogenki desu-ka???
genki desu...
yoroshiku onegaishimasu...
Surechigau shoujotachi
Mabushikute me wo sorashita
Mujaki na mama no kodomo no you na
Jiyuu na hane wo motte ita
Kurayami no mukougawa
Hikari sasu basho wo motome
Hitotsu nokotta tsubasa hirogetemo
Shinjitsu ni dake todokanai
Soko kara miru watashi no sugata wa
Donna fuu ni utsutte imasu ka
Konna konna itsuwari darake no
Hibi wo waraitobashite kudasai
Teokure ni naru sono mae ni
Tobu koto ni tsukaretemo
Hane orosu yuuki mo nai
Moshimo negai ga hitotsu kanau nara
Isso koko kara tsuredashite
Ima mo koko de watashi wa kawarazu
Ibasho wo zutto sagashite imasu
Douka douka anata ni dake wa
Kono omoi ga tsutawarimasu you ni
Hoshii mono nado hoka ni nai
Soko kara miru watashi no sugata wa
Donna fuu ni utsutte imasu ka
Konna konna itsuwari darake no
Hibi wo waraitobashite kudasai
Ima mo koko de watashi wa kawarazu
Ibasho wo zutto sagashite imasu
Douka douka anata ni dake wa
Kono omoi ga tsutawarimasu you ni
Hoshii mono nado hoka ni nai
Kimi wo aishita hibi wa
Boku no saigo no kiseki
Daremo ga mina
Hito koishiku naru kisetsu ga
Kotoshi mo mata
Atataka sa to tsumeta sa wo
Tsurete yatte kita
Osana sugita bokura ga mada
Nanimo shirazu
Warai atte shigami tsuite
Aruiteita hi wo
Omoidasu
Oshi yoseru konna itami ni
Donna ii wake wo sureba ii
Tsukare hateta karada de Nemuri ni tsuita kimi wo
Boku wa iki wo hisomete Mite ita
Sekaijuu de Tada hitori Boku dake ga shitte iru
Muboubi de Itoshii Yokogao
Meguri aeta toki wo omou
Onaji kisetsu mezameru you ni
Hateshinai itoshisa wa kono
Kokoro ni tashika ni umareru
Kagayaki no hitotsu to shite bokura wa
Hoshi tachi no shita de rekishi wo kizamu
Atashi wa....
Label: At the moment...
DNA And Chtomosome...

DNA 3D... Double helix...
DNA...
DNA Structure...
DNA Replication...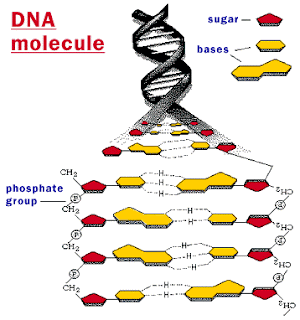
DNA Structure...
Human Chromosome... There are 46 chromosome.. 44 autosome and 2 gonosome...
Label: Knowledge
Biology...

Animal Cell.... I'ts an animal cell...
Eritrosit... Sel darah merah...
Escherichia Coli...
Vibrio Cholerae...
Anthrax Vyruses.......
Label: Knowledge
Ebola Disease...

Ni adl bentuk dari virus ebola.... sampai saat ini belum ada obat untuk mengobati penyalit ini... vaksin pun juga blm ada... virus ini sangat cepat dalam menyerang organ" tubuh... biasany, pasien hanya akan bertahan hidup selama beberapa hari.... maksimal hanya7-9 hari sebelum ajal menjemput.... ketika berhasil bertahan lebih dari 5 hari, pasien akan muntah darah dan memuntahkan organ tubuhny yang telah hancur oleh serangan virus ini... penyakit ini ditemukan di Afrika.... sungguh mengerikan!!!
Salah satu rumah sakit isolasi untuk pasien ebola di Afrika...
Ebola
Ebola is the common term for a group of viruses belonging to genus Ebolavirus, family Filoviridae, and for the disease which they cause, Ebola hemorrhagic fever. The virus is named after the Ebola River where the first recognised outbreak of Ebola hemorrhagic fever occurred. The viruses are characterized by long filaments and have a similar shape to the Marburg virus, also in the family Filoviridae, and share similar disease symptoms. Since its discovery, Ebolavirus has been responsible for a number of deaths.[1]
Ebolavirus first came to notice in 1976 in outbreaks of Ebola hemorrhagic fever in Zaire and Sudan.[2] The strain of Ebola which broke out in
Ebola is believed to be a zoonotic virus as it is currently devastating the populations of Western Lowland Gorillas in Central Africa. As of late 2005, three species of fruit bat have been identified as carrying the virus but not showing disease symptoms, and they are now believed to be the natural host species, or reservoir, of the virus.[citation needed]
Ebola hemorrhagic fever is potentially lethal and encompasses a range of symptoms including fever, vomiting, diarrhea, generalized pain or malaise, and sometimes internal and external bleeding. Mortality rates are extremely high, with the human case-fatality rate ranging from 50–89%, depending on viral subtype.[5] The cause of death is usually due to hypovolemic shock or organ failure.
Ebola is potentially lethal, and since no approved vaccine or treatment is available, Ebola is classified as a biosafety level 4 agent, as well as a Category A bioterrorism agent by the Centers for Disease Control and Prevention. It has the potential to be weaponized for use in biological warfare.[6] Its effectiveness as a biological-warfare agent is compromised by its extreme deadliness and its level of contagion: a typical outbreak spreads through a small village or hospital, infects the entire population, and then runs out of potential hosts, dying out before reaching the wider community. It is also significant that none of the strains of Ebola known to cause disease in humans have been found to be airborne—only the strain known as Ebola Reston (after the city of Reston, Virginia where it was first identified in Green Monkeys) is believed to be airborne.
The virus is named after the Ebola River Valley in the Democratic Republic of the Congo (formerly Zaïre), which is near the site of the first recognized outbreak in 1976, in a mission hospital run by Flemish nuns.[7]
Electron micrographs of members of genus Ebolavirus show them to have the characteristic thread-like structure of a filovirus.[8] EBOV VP30 is around 288 amino acids long.[8] The virions are tubular in general form but variable in overall shape and may appear as the classic shepherd's crook or eyebolt, as a U or a 6, or coiled, circular, or branched. However, laboratory purification techniques such as centrifugation may contribute to some of these.[8] Virions are generally 80 nm in diameter.[8] They are of variable length, typically around 1000 nm, but may be up to 1400 nm long. In the center of the virion is a structure called nucleocapsid, which is formed by the helically wound viral genomic RNA complexed with the proteins NP, VP35, VP30 and L. It has a diameter of 40-50 nm and contains a central channel of 20–30 nm in diameter. Virally encoded glycoprotein (GP) spikes 10 nm long and 10 nm apart are present on the outer viral envelope of the virion, which is derived from the host cell membrane. Between envelope and nucleocapsid, in the so-called matrix space, the viral proteins VP40 and VP24 are located..
Each virion contains one minor molecule of linear, single-stranded, negative-sense RNA, totaling 18,959 to 18,961 nucleotides in length. The 3′ terminus is not polyadenylated and the 5′ end is not capped. It was found that 472 nucleotides from the 3' end and 731 nucleotides from the 5' end were sufficient for replication.[8] It codes for seven structural proteins and one non-structural protein. The gene order is 3′ - leader - NP - VP35 - VP40 - GP/sGP - VP30 - VP24 - L - trailer - 5′; with the leader and trailer being non-transcribed regions which carry important signals to control transcription, replication and packaging of the viral genomes into new virions. The genomic material by itself is not infectious, because viral proteins, among them the RNA-dependent RNA polymerase, are necessary to transcribe the viral genome into mRNAs, as well as for replication of the viral genome.
- Virus attaches to host receptors through the GP (glycoprotein) surface peplomer and is endocytosed into vesicles in the host cell.
- Fusion of virus membrane with the vesicle membrane occurs; nucleocapsid is released into the cytoplasm.
- The encapsidated, negative-sense genomic ssRNA is used as a template for the synthesis ( 3' - 5') of polyadenylated, monocistronic mRNAs.
- Translation of the mRNA into viral proteins occurs using the host cell's machinery.
- Post-translational processing of viral proteins occurs. GP0 (glycoprotein precursor) is cleaved to GP1 and GP2, which are heavily glycosylated. These two molecules assemble, first into heterodimers, and then into trimers to give the surface peplomers. SGP (secreted glycoprotein) precursor is cleaved to SGP and delta peptide, both of which are released from the cell.
- As viral protein levels rise, a switch occurs from translation to replication. Using the negative-sense genomic RNA as a template, a complementary +ssRNA is synthesized; this is then used as a template for the synthesis of new genomic (-)ssRNA, which is rapidly encapsidated.
- The newly formed nucleocapsides and envelope proteins associate at the host cell's plasma membrane; budding occurs, and the virions are released.
Despite numerous studies, the wildlife reservoir of Ebolavirus has not been identified. Between 1976 and 1998, from 30,000 mammals, birds, reptiles, amphibians, and arthropods sampled from outbreak regions, no Ebolavirus was detected[9] apart from some genetic material found in six rodents (Mus setulosus and Praomys species) and a shrew (Sylvisorex ollula) collected from the Central African Republic in 1998.[10] Ebolavirus was detected in the carcasses of gorillas, chimpanzees and duikers during outbreaks in 2001 and 2003 (the carcasses were the source of the initial human infections), but the high mortality from infection in these species precludes them from acting as reservoirs.[9]
Plants, arthropods, and birds have also been considered as reservoirs; however, bats are considered the most likely candidate.[11] Bats were known to reside in the cotton factory in which the index cases for the 1976 and 1979 outbreaks were employed and have also been implicated in Marburg infections in 1975 and 1980.[9] Of 24 plant species and 19 vertebrate species experimentally inoculated with Ebolavirus, only bats became infected.[12] The absence of clinical signs in these bats is characteristic of a reservoir species. In 2002-03, a survey of 1,030 animals from Gabon and the Republic of the Congo including 679 bats found Ebolavirus RNA in 13 fruit bats (Hyspignathus monstrosus, Epomops franquetti and Myonycteris torquata).[13] Bats are also known to be the reservoirs for a number of related viruses including Nipah virus, Hendra virus and lyssaviruses.
Microbiologists have defined several subtypes of Ebola. The following list is not exclusive. A new strain of Ebolavirus has been identified in Uganda during an outbreak. It does not match any of the four Ebola subtypes previously identified by scientists.[14]
The Zaïre ebolavirus has the highest case fatality rate, up to 90% in some epidemics, with an average case fatality rate of approximately 83% over 27 years. The case-fatality rates were 88% in 1976, 100% in 1977, 59% in 1994, 81% in 1995, 73% in 1996, 80% in 2001-2002 and 90% in 2003. There have been more outbreaks of Zaïre ebolavirus than any other strain.
The first outbreak took place on August 26, 1976, in Yambuku, a town in the north of Zaïre. The first recorded case was Mabalo Lokela, a 44-year-old schoolteacher returning from a trip around the north of the state. His high fever was diagnosed as possible malaria and he was subsequently given a quinine shot. Lokela returned to the hospital every day. A week later, his symptoms included uncontrolled vomiting, bloody diarrhea, headache, dizziness, and trouble breathing. Later, he began bleeding from his nose, mouth, and anus. Lokela died on September 8, 1976, roughly 14 days after the onset of symptoms.
Soon after, more patients arrived with varying but similar symptoms including fever, headache, muscle and joint aches, fatigue, nausea, and dizziness. These often progressed to bloody diarrhea, severe vomiting, and bleeding from the nose, mouth, and anus. The initial transmission was believed to be due to reuse of the needle for Lokela's injection without sterilization. Subsequent transmission was also due to care of the sick patients without barrier nursing and the traditional burial preparation method, which involved washing and gastrointestinal tract cleansing.
Two nuns working in Yambuku as nurses also died in the same outbreak.[15]
Sudan ebolavirus was the second strain of Ebola reported in 1976. It apparently originated amongst cotton factory workers in Nzara, Sudan. The first case reported was a worker exposed to a potential natural reservoir at the cotton factory. Scientists tested all animals and insects in response to this, however none tested positive for the virus. The carrier is still unknown.
A second case involved a nightclub owner in Nzara, Sudan. The local hospital, Maridi, tested and attempted to treat the patient; however, nothing was successful, and he died. The hospital did not advocate safe and practical procedures in sterilizing and disinfecting the medical tools used on the nightclub owner, likely facilitating the spread of the virus in the hospital.
The most recent outbreak of Sudan ebolavirus occurred in May 2004. As of May 2004, 20 cases of Sudan ebolavirus were reported in Yambio County, Sudan, with five deaths resulting. The Centers for Disease Control and Prevention confirmed the virus a few days later. The neighbouring countries of Uganda and the Democratic Republic of Congo have increased surveillance in bordering areas, and other similar measures have been taken to control the outbreak. The average fatality rates for Sudan ebolavirus were 54% in 1976, 68% in 1979, and 53% in 2000/2001. The average case-fatality rate is 54%.
The Reston ebolavirus is suspected of being either another subtype of the Ebola or a new filovirus of Asian origin. It was discovered in crab-eating macaques from Hazleton Labortories (now Covance) in 1989. This discovery attracted significant media attention and led to the publication of The Hot Zone. Despite its status as a Level-4 organism, the Reston ebolavirus is non-pathogenic to humans and is only mildly fatal to monkeys;[16] the perception of its lethality was skewed due to the monkey's coinfection with Simian hemorrhagic fever virus (SHFV).[17]
During the incident in which it was discovered, six animal handlers eventually became seroconverted, one of whom had cut himself while performing a necropsy on the liver of an infected monkey. When the handler failed to become ill, it was concluded that the virus had a very low pathogenicity to humans.[18] Monkeys infected with Reston ebolavirus were again shipped to Reston, as well as Alice, Texas, in February 1990. More cases of Reston ebolavirus-infected monkeys were discovered in Siena, Italy in 1992, and in Texas again in March 1996.
This subtype of Ebola was first discovered amongst chimpanzees of the Tai Forest in Côte d'Ivoire, Africa. On November 1, 1994, the corpses of two chimpanzees were found in the forest. Necropsies showed blood within the heart to be liquid and brown, no obvious marks seen on the organs, and one with lungs filled with liquid blood. Studies of tissues taken from the chimps showed results similar to human cases during the 1976 Ebola outbreaks in Zaïre and Sudan. Later in 1994, more dead chimpanzees were discovered, with many testing positive to Ebola using molecular techniques. The source of contamination was believed to be the meat of infected Western Red Colobus monkeys, upon which the chimpanzees preyed.[19]
One of the scientists performing the necropsies on the infected chimpanzees contracted Ebola. She developed symptoms similar to dengue fever approximately a week after the necropsy and was transported to Switzerland for treatment. After two weeks she was discharged from hospital, and was fully recovered six weeks after the infection.
On November 24, 2007, the Uganda Ministry of Health confirmed an outbreak of Ebola in the Bundibugyo District. After confirmation of samples tested by the United States National Reference Laboratories and the Centers for Disease Control, the World Health Organization confirmed the presence of a new species of Ebolavirus. On February 20, 2008, the Uganda Ministry officially announced the end of the epidemic in Bundibugyo with the last infected person discharged on January 8, 2008.[20] Ugandan officials confirmed a total of 149 cases of this new Ebola species, with 37 deaths attributed to the strain.[21]
Symptoms are varied and often appear suddenly. Initial symptoms include high fever (at least 38.8°C; 101.8°F), severe headache, muscle, joint, or abdominal pain, severe weakness and exhaustion, sore throat, nausea, and dizziness.[22] Before an outbreak is suspected, these early symptoms are easily mistaken for malaria, typhoid fever, dysentery, influenza, or various bacterial infections, which are all far more common and reliably less fatal.
Ebola may progress to cause more serious symptoms, such as diarrhea, dark or bloody feces, vomiting blood, red eyes due to distension and hemorrhage of sclerotic arterioles, petechia, maculopapular rash, and purpura. Other secondary symptoms include hypotension (low blood pressure), hypovolemia, tachycardia, organ damage (especially the kidneys, spleen, and liver) as a result of disseminated systemic necrosis, and proteinuria. The interior bleeding is caused by a reaction between the virus and the platelets that produces a chemical that will cut cell-sized holes into the capillary walls.
Occasionally, internal and external hemorrhage from orifices, such as the nose and mouth, may also occur, as well as from incompletely healed injuries such as needle-puncture sites. Ebola virus can affect the levels of white blood cells and platelets, disrupting clotting.[citation needed] More than 50% of patients will develop some degree of hemorrhaging.[citation needed]
Methods of diagnosis of Ebola include testing saliva and urine samples. The span of time from onset of symptoms to death is usually between 2 and 21 days. By the second week of infection, patients will either defervesce (the fever will lessen) or undergo systemic multi-organ failure. Mortality rates are generally high, ranging from 50 to 90%.[22] The cause of death is usually due to hypovolemic shock or organ failure.[23]
Filoviruses replicate well in a wide range of organs and cell types such as hepatocytes, epithelial cells, fibroblasts, fibroblastic reticular cells and adrenal cortical cells.[8] Most notably, the susceptibility of human endothelial cells is likely the cause of the symptoms that appear in the late stages of the infection such as shock syndrome and hemorrhaging.[8]
Among humans, the virus is transmitted by direct contact with infected body fluids or, to a lesser extent, skin or mucous membrane contact. The incubation period can range from 2 to 21 days, but is generally 5–10 days.
Although airborne transmission between monkeys has been demonstrated by an accidental outbreak in a laboratory located in Virginia, USA, there is very limited evidence for human-to-human airborne transmission in any reported epidemics. Nurse Mayinga might represent the only possible case. The means by which she contracted the virus remains uncertain.
The infection of human cases with Ebolavirus has been documented through the handling of infected chimpanzees, gorillas, and forest antelopes—both dead and alive—as was documented in Côte d'Ivoire, the Republic of Congo and Gabon. The transmission of the Ebola Reston strain through the handling of cynomolgus monkeys has also been reported.[22]
So far, all epidemics of Ebola have occurred in sub-optimal hospital conditions, where practices of basic hygiene and sanitation are often either luxuries or unknown to caretakers and where disposable needles and autoclaves are unavailable or too expensive. In modern hospitals with disposable needles and knowledge of basic hygiene and barrier nursing techniques, Ebola has never spread on such a large scale.
In the early stages, Ebola may not be highly contagious. Contact with someone in early stages may not even transmit the disease. As the illness progresses, bodily fluids from diarrhea, vomiting, and bleeding represent an extreme biohazard. Due to lack of proper equipment and hygienic practices, large scale epidemics occur mostly in poor, isolated areas without modern hospitals or well-educated medical staff. Many areas where the infectious reservoir exists have just these characteristics. In such environments, all that can be done is to immediately cease all needle-sharing or use without adequate sterilization procedures, to isolate patients, and to observe strict barrier nursing procedures with the use of a medical rated disposable face mask, gloves, goggles, and a gown at all times. This should be strictly enforced for all medical personnel and visitors.
Ebola is unlikely to develop into a pandemic, or world-wide infection, due to its difficulty in spreading by airborne transmission and the period of time that the virus can use a living and contagious victim to spread compared to other infectious diseases. In isolated settings such as a quarantined hospital or a remote village, most victims are infected shortly after the first case of infection is present. In addition, the quick onset of symptoms from the time the disease becomes contagious in an individual makes it easy to identify sick individuals and limits an individual's ability to spread the disease by traveling. Because bodies of the deceased are still infectious, some doctors had to take measures to make sure that the disposal of dead bodies were conducted in a safe manner despite any local traditional burial rituals.[24]
There is no standard treatment for Ebola HF. Treatment is primarily supportive and includes minimizing invasive procedures, balancing electrolytes since patients are frequently dehydrated, replacing lost coagulation factors to help stop bleeding, maintaining oxygen and blood levels, and treating any complicating infections. Convalescent plasma (factors from those who have survived Ebola infection) shows promise as a treatment for the disease[citation needed]. Ribavirin is ineffective. Interferon is also thought to be ineffective. In monkeys, administration of an inhibitor of coagulation (rNAPc2) has shown some benefit, protecting 33% of infected animals from a usually 100% (for monkeys) lethal infection (unfortunately, this inoculation does not work on humans). In early 2006, scientists at USAMRIID announced a 75% recovery rate after infecting four rhesus monkeys with Ebolavirus and administering antisense drugs.[25]
Vaccines have been produced for both Ebola[26] and Marburg[27] that were 99% effective in protecting a group of monkeys from the disease. These vaccines are based on either a recombinant Vesicular stomatitis virus or a recombinant Adenovirus[28] carrying the Ebola spikeprotein on its surface. Early human vaccine efforts, like the one at NIAID in 2003, have so far not reported any successes.[29] The biggest problem with the vaccine is that unless the patient is given it near the onset of the virus (1-4 days after the symptoms begin), then there will be too much damage to the human body to repair, e.g., ruptured arteries and capillaries, vomiting, and other symptoms which may still cause enough harm to kill or seriously traumatize the patient.
As of August 30, 2007, 103 people (100 adults and three children) were infected by a suspected hemorrhagic fever outbreak in the village of Kampungu, Democratic Republic of the Congo (DRC). The outbreak started after the funerals of two village chiefs, and 217 people in four villages fell ill. The World Health Organization sent a team to take blood samples for analysis and confirmed that many of the cases are the result of Ebolavirus.[30] The Congo's last major Ebola epidemic killed 245 people in 1995 in Kikwit, about 200 miles from the source of the August 2007 outbreak.[31]
On November 30, 2007, the Uganda Ministry of Health confirmed an outbreak of Ebola in the Bundibugyo District. After confirmation of samples tested by the United States National Reference Laboratories and the Centers for Disease Control, the World Health Organization confirmed the presence of a new species of Ebolavirus.[32] The epidemic came to an official end on February 20, 2008. While it lasted, 149 cases of this new strain were reported, and 37 of those led to deaths.
Because of Ebola lethality and lack of approved vaccine or treatment, Ebola is classified as a Biosafety Level 4 agent, as well as a Category A bioterrorism agent[33] and a select agent by the CDC.
As a terrorist weapon, Ebola has been considered by members of Japan's Aum Shinrikyo cult, whose leader, Shoko Asahara, led about 40 members to Zaire in 1992 under the guise of offering medical aid to Ebola victims in what was presumably an attempt to acquire a sample of the virus.
Label: Knowledge
Albert Einstein.....
Albert Einstein
Albert Einstein (14 Maret 1879–18 April 1955) adalah seorang ilmuwan fisika teoretis yang dipandang luas sebagai ilmuwan terbesar dalam abad ke-20. Dia mengemukakan teori relativitas dan juga banyak menyumbang bagi pengembangan mekanika kuantum, mekanika statistik, dan kosmologi. Dia dianugerahi Penghargaan Nobel dalam Fisika pada tahun 1921 untuk penjelasannya tentang efek fotoelektrik dan "pengabdiannya bagi Fisika Teoretis".
Setelah teori relativitas umum dirumuskan, Einstein menjadi terkenal ke seluruh dunia, pencapaian yang tidak biasa bagi seorang ilmuwan. Di masa tuanya, keterkenalannya melampaui ketenaran semua ilmuwan dalam sejarah, dan dalam budaya populer, kata Einstein dianggap bersinonim dengan kecerdasan atau bahkan jenius. Wajahnya merupakan salah satu yang paling dikenal di seluruh dunia.
Pada tahun 1999, Einstein dinamakan "Tokoh Abad Ini" oleh majalah Time. Kepopulerannya juga membuat nama "Einstein" digunakan secara luas dalam iklan dan barang dagangan lain, dan akhirnya "Albert Einstein" didaftarkan sebagai merk dagang.
Untuk menghargainya, sebuah satuan dalam fotokimia dinamai einstein, sebuah unsur kimia dinamai einsteinium, dan sebuah asteroid dinamai 2001 Einstein.
Rumus Einstein yang paling terkenal adalah E=mc²
Einstein dilahirkan di Ulm di Württemberg, Jerman; sekitar 100 km sebelah timur Stuttgart. Bapaknya bernama Hermann Einstein, seorang penjual ranjang bulu yang kemudian menjalani pekerjaan elektrokimia, dan ibunya bernama Pauline. Mereka menikah di
Pada umur lima tahun, ayahnya menunjukkan kompas kantung, dan Einstein menyadari bahwa sesuatu di ruang yang "kosong" ini beraksi terhadap jarum di kompas tersebut; dia kemudian menjelaskan pengalamannya ini sebagai salah satu saat yang paling menggugah dalam hidupnya. Meskipun dia membuat model dan alat mekanik sebagai hobi, dia dianggap sebagai pelajar yang lambat, kemungkinan disebabkan oleh dyslexia, sifat pemalu, atau karena struktur yang jarang dan tidak biasa pada otaknya (diteliti setelah kematiannya). Dia kemudian diberikan penghargaan untuk teori relativitasnya karena kelambatannya ini, dan berkata dengan berpikir dalam tentang ruang dan waktu dari anak-anak lainnya, dia mampu mengembangkan kepandaian yang lebih berkembang. Pendapat lainnya, berkembang belakangan ini, tentang perkembangan mentalnya adalah dia menderita Sindrom Asperger, sebuah kondisi yang berhubungan dengan autisme.
Einstein mulai belajar matematika pada umur dua belas tahun. Ada gosip bahwa dia gagal dalam matematika dalam jenjang pendidikannya, tetapi ini tidak benar; penggantian dalam penilaian membuat bingung pada tahun berikutnya. Dua pamannya membantu mengembangkan ketertarikannya terhadap dunia intelek pada masa akhir kanak-kanaknya dan awal remaja dengan memberikan usulan dan buku tentang sains dan matematika.
Pada tahun 1894, dikarenakan kegagalan bisnis elektrokimia ayahnya, Einstein pindah dari Munich ke Pavia, Italia (dekat kota Milan). Albert tetap tinggal untuk menyelesaikan sekolah, menyelesaikan satu semester sebelum bergabung kembali dengan keluarganya di Pavia.
Kegagalannya dalam seni liberal dalam tes masuk Eidgenössische Technische Hochschule (Institut Teknologi Swiss Federal, di Zurich) pada tahun berikutnya adalah sebuah langkah mundur dia oleh keluarganya dikirim ke Aarau, Swiss, untuk menyelesaikan sekolah menengahnya, di mana dia menerima diploma pada tahun 1896, Einstein beberapa kali mendaftar di Eidgenössische Technische Hochschule. Pada tahun berikutnya dia melepas kewarganegaraan Württemberg, dan menjadi tak bekewarganegaraan.
Pada 1898, Einstein menemui dan jatuh cinta kepada Mileva Marić, seorang Serbia yang merupakan teman kelasnya (juga teman Nikola Tesla). Pada tahun 1900, dia diberikan gelar untuk mengajar oleh Eidgenössische Technische Hochschule dan diterima sebagai warga negar Swiss pada 1901. Selama masa ini Einstein mendiskusikan ketertarikannya terhadap sains kepada teman-teman dekatnya, termasuk Mileva. Dia dan Mileva memiliki seorang putri bernama Lieserl, lahir dalam bulan Januari tahun 1902. Lieserl Einstein, pada waktu itu, dianggap tidak legal karena orang tuanya tidak menikah.
Pada saat kelulusannya Einstein tidak dapat menemukan pekerjaan mengajar, keterburuannya sebagai orang muda yang mudah membuat marah professornya. Ayah seorang teman kelas menolongnya mendapatkan pekerjaan sebagai asisten teknik pemeriksa di Kantor Paten Swiss pada tahun 1902. Di
Einstein menikahi Mileva pada 6 Januari 1903. Pernikahan Einstein dengan Mileva, seorang matematikawan. Pada 14 Mei 1904, anak pertama dari pasangan ini, Hans Albert Einstein, lahir. Pada 1904, posisi Einstein di Kantor Paten Swiss menjadi tetap. Dia mendapatkan gelar doktor setelah menyerahkan thesis "Eine neue Bestimmung der Moleküldimensionen" ("On a new determination of molecular dimensions") pada tahun 1905 dari Universitas Zürich.
Di tahun yang sama dia menulis empat artikel yang memberikan dasar fisika modern, tanpa banyak sastra sains yang dapat ia tunjuk atau banyak kolega dalam sains yang dapat ia diskusikan tentang teorinya. Banyak fisikawan setuju bahwa ketiga thesis itu (tentang gerak Brownian), efek fotolistrik, dan relativitas khusus) pantas mendapat Penghargaan Nobel. Tetapi hanya thesis tentang efek fotoelektrik yang mendapatkan penghargaan tersebut. Ini adalah sebuah ironi, bukan hanya karena Einstein lebih tahu banyak tentang relativitas, tetapi juga karena efek fotoelektrik adalah sebuah fenomena kuantum, dan Einstein menjadi terbebas dari jalan dalam teori kuantum. Yang membuat thesisnya luar biasa adalah, dalam setiap kasus, Einstein dengan yakin mengambil ide dari teori fisika ke konsekuensi logis dan berhasil menjelaskan hasil eksperimen yang membingungkan para ilmuwan selama beberapa dekade.
Dia menyerahkan thesis-thesisnya ke "Annalen der Physik". Mereka biasanya ditujukan kepada "Annus Mirabilis Papers" (dari Latin: Tahun luar biasa). Persatuan Fisika Murni dan Aplikasi (IUPAP) merencanakan untuk merayakan 100 tahun publikasi pekerjaan Einstein di tahun 1905 sebagai Tahun Fisika 2005.
Di artikel pertamanya di tahun 1905 bernama "On the Motion—Required by the Molecular Kinetic Theory of Heat—of Small Particles Suspended in a Stationary Liquid", mencakup penelitian tentang gerakan Brownian. Menggunakan teori kinetik cairan yang pada saat itu kontroversial, dia menetapkan bahwa fenomena, yang masih kurang penjelasan yang memuaskan setelah beberapa dekade setelah ia pertama kali diamati, memberikan bukti empirik (atas dasar pengamatan dan eksperimen) kenyataan pada atom. Dan juga meminjamkan keyakinan pada mekanika statistika, yang pada saat itu juga kontroversial.
Sebelum thesis ini, atom dikenal sebagai konsep yang berguna, tetapi fisikawan dan kimiawan berdebat dengan sengit apakah atom itu benar-benar suatu benda yang nyata. Diskusi statistik Einstein tentang kelakuan atom memberikan pelaku eksperimen sebuah cara untuk menghitung atom hanya dengan melihat melalui mikroskop biasa. Wilhelm Ostwald, seorang pemimpin sekolah anti-atom, kemudian memberitahu Arnold Sommerfeld bahwa ia telah berkonversi kepada penjelasan komplit Einstein tentang gerakan Brown.
Label: Knowledge
Cancer Disease...

Ni dy pembelahan sel kanker.... begitu cepat dan begitu banyak... Ni kanker yg menyerang usus besar...
Ni kanker yg menyerang usus besar...
Ni adl kanker paru"..... sel kanker yg menyerang paru" menyebabkan jadi deperti ini....
Hey!!! do u know cancer disease??????
yepz..... cancer is one of the most danger diseases......
want to know about cancer?????
let's read about cancer below.....
Cancer
Cancer (medical term: malignant neoplasm) is a class of diseases in which a group of cells display uncontrolled growth (division beyond the normal limits), invasion (intrusion on and destruction of adjacent tissues), and sometimes metastasis (spread to other locations in the body via lymph or blood). These three malignant properties of cancers differentiate them from benign tumors, which are self-limited, do not invade or metastasize. Most cancers form a tumor but some, like leukemia, do not. The branch of medicine concerned with the study, diagnosis, treatment, and prevention of cancer is oncology.
Cancer may affect people at all ages, even fetuses, but the risk for most varieties increases with age.[1] Cancer causes about 13% of all deaths.[2] According to the American Cancer Society, 7.6 million people died from cancer in the world during 2007.[3] Cancers can affect all animals.
Nearly all cancers are caused by abnormalities in the genetic material of the transformed cells. These abnormalities may be due to the effects of carcinogens, such as tobacco smoke, radiation, chemicals, or infectious agents. Other cancer-promoting genetic abnormalities may be randomly acquired through errors in DNA replication, or are inherited, and thus present in all cells from birth. The heritability of cancers are usually affected by complex interactions between carcinogens and the host's genome. New aspects of the genetics of cancer pathogenesis, such as DNA methylation, and microRNAs are increasingly recognized as important.
Genetic abnormalities found in cancer typically affect two general classes of genes. Cancer-promoting oncogenes are typically activated in cancer cells, giving those cells new properties, such as hyperactive growth and division, protection against programmed cell death, loss of respect for normal tissue boundaries, and the ability to become established in diverse tissue environments. Tumor suppressor genes are then inactivated in cancer cells, resulting in the loss of normal functions in those cells, such as accurate DNA replication, control over the cell cycle, orientation and adhesion within tissues, and interaction with protective cells of the immune system.
Diagnosis usually requires the histologic examination of a tissue biopsy specimen by a pathologist, although the initial indication of malignancy can be symptoms or radiographic imaging abnormalities. Most cancers can be treated and some cured, depending on the specific type, location, and stage. Once diagnosed, cancer is usually treated with a combination of surgery, chemotherapy and radiotherapy. As research develops, treatments are becoming more specific for different varieties of cancer. There has been significant progress in the development of targeted therapy drugs that act specifically on detectable molecular abnormalities in certain tumors, and which minimize damage to normal cells. The prognosis of cancer patients is most influenced by the type of cancer, as well as the stage, or extent of the disease. In addition, histologic grading and the presence of specific molecular markers can also be useful in establishing prognosis, as well as in determining individual treatments.
Cancer is generally classified according to the tissue from which the cancerous cells originate, the primary tumor, as well as the normal cell type they most resemble. These are location and histology, respectively.
Nomenclature
The following closely related terms may be used to designate abnormal growths:
- Tumor or tumour: originally, it meant any abnormal swelling, lump or mass. In current English, however, the word tumor has become synonymous with neoplasm, specifically solid neoplasm. Note that some neoplasms, such as leukemia, do not form tumors.
- Neoplasm: the scientific term to describe an abnormal proliferation of genetically altered cells. Neoplasms can be benign or malignant:
- Malignant neoplasm or malignant tumor: synonymous with cancer.
- Benign neoplasm or benign tumor: a tumor (solid neoplasm) that stops growing by itself, does not invade other tissues and does not form metastases.
- Invasive tumor is another synonym of cancer. The name refers to invasion of surrounding tissues.
- Pre-malignancy, pre-cancer or non-invasive tumor: A neoplasm that is not invasive but has the potential to progress to cancer (become invasive) if left untreated. These lesions are, in order of increasing potential for cancer, atypia, dysplasia and carcinoma in situ.
The following terms can be used to describe a cancer:
- Screening: a test done on healthy people to detect tumors before they become apparent. A mammogram is a screening test.
- Diagnosis: the confirmation of the cancerous nature of a lump. This usually requires a biopsy or removal of the tumor by surgery, followed by examination by a pathologist.
- Surgical excision: the removal of a tumor by a surgeon.
- Surgical margins: the evaluation by a pathologist of the edges of the tissue removed by the surgeon to determine if the tumor was removed completely ("negative margins") or if tumor was left behind ("positive margins").
- Grade: a number (usually on a scale of 3) established by a pathologist to describe the degree of resemblance of the tumor to the surrounding benign tissue.
- Stage: a number (usually on a scale of 4) established by the oncologist to describe the degree of invasion of the body by the tumor.
- Recurrence: new tumors that appear at the site of the original tumor after surgery.
- Metastasis: new tumors that appear far from the original tumor.
- Transformation: the concept that a low-grade tumor transforms to a high-grade tumor over time. Example: Richter's transformation.
- Chemotherapy: treatment with drugs.
- Radiation therapy: treatment with radiations.
- Adjuvant therapy: treatment, either chemotherapy or radiation therapy, given after surgery to kill the remaining cancer cells.
- Prognosis: the probability of cure after the therapy. It is usually expressed as a probability of survival five years after diagnosis. Alternatively, it can be expressed as the number of years when 50% of the patients are still alive. Both numbers are derived from statistics accumulated with hundreds of similar patients to give a Kaplan-Meier curve.
Cancers are classified by the type of cell that resembles the tumor and, therefore, the tissue presumed to be the origin of the tumor. Examples of general categories include:
- Carcinoma: Malignant tumors derived from epithelial cells. This group represents the most common cancers, including the common forms of breast, prostate, lung and colon cancer.
- Sarcoma: Malignant tumors derived from connective tissue, or mesenchymal cells.
- Lymphoma and leukemia: Malignancies derived from hematopoietic (blood-forming) cells
- Germ cell tumor: Tumors derived from totipotent cells. In adults most often found in the testicle and ovary; in fetuses, babies, and young children most often found on the body midline, particularly at the tip of the tailbone; in horses most often found at the poll (base of the skull).
- Blastic tumor or blastoma: A tumor (usually malignant) which resembles an immature or embryonic tissue. Many of these tumors are most common in children.
Malignant tumors (cancers) are usually named using -carcinoma, -sarcoma or -blastoma as a suffix, with the Latin or Greek word for the organ of origin as the root. For instance, a cancer of the liver is called hepatocarcinoma; a cancer of the fat cells is called liposarcoma. For common cancers, the English organ name is used. For instance, the most common type of breast cancer is called ductal carcinoma of the breast or mammary ductal carcinoma. Here, the adjective ductal refers to the appearance of the cancer under the microscope, resembling normal breast ducts.
Benign tumors (which are not cancers) are named using -oma as a suffix with the organ name as the root. For instance, a benign tumor of the smooth muscle of the uterus is called leiomyoma (the common name of this frequent tumor is fibroid). Unfortunately, some cancers also use the -oma suffix, examples being melanoma and seminoma.
Adult cancers
In the U.S. and other developed countries, cancer is presently responsible for about 25% of all deaths.[4] On a yearly basis, 0.5% of the population is diagnosed with cancer. The statistics below are for adults in the United States, and may vary substantially in other countries:
| Male | | Female | ||
| most common (by occurrence) | most common (by mortality)[4] | most common (by occurrence) | most common (by mortality)[4] | |
| prostate cancer (33%) | lung cancer (31%) | breast cancer (32%) | lung cancer (27%) | |
| lung cancer (13%) | prostate cancer (10%) | lung cancer (12%) | breast cancer (15%) | |
| colorectal cancer (10%) | colorectal cancer (10%) | colorectal cancer (11%) | colorectal cancer (10%) | |
| bladder cancer (7%) | pancreatic cancer (5%) | endometrial cancer (6%) | ovarian cancer (6%) | |
| cutaneous melanoma (5%) | leukemia (4%) | non-Hodgkin lymphoma (4%) | pancreatic cancer (6%) | |
Child cancers
Cancer can also occur in young children and adolescents, but it is rare (about 150 cases per million yearly in the US). Statistics from the SEER program of the US NCI demonstrate that childhood cancers increased 19% between 1975 and 1990, mainly due to an increased incidence in acute leukemia. Since 1990, incidence rates have decreased.[5]
Children living near nuclear facilities face an increased risk of cancer.[6]
Infant cancers
The age of peak incidence of cancer in children occurs during the first year of life, in infants. The average annual incidence in the United States, 1975-1995, was 233 per million infants.[5] Several estimates of incidence exist. According to SEER,[5] in the United States:
- Neuroblastoma comprised 28% of infant cancer cases and was the most common malignancy among these young children (65 per million infants).
- The leukemias as a group (41 per million infants) represented the next most common type of cancer, comprising 17% of all cases.
- Central nervous system malignancies comprised 13% of infant cancer, with an average annual incidence rate of nearly 30 per million infants.
- The average annual incidence rates for malignant germ cell and malignant soft tissue tumors were essentially the same at 15 per million infants. Each comprised about 6% of infant cancer.
According to another study:[4]
- Leukemia (usually ALL) is the most common infant malignancy (30%), followed by the central nervous system cancers and neuroblastoma. The remainder consists of Wilms' tumor, lymphomas, rhabdomyosarcoma (arising from muscle), retinoblastoma, osteosarcoma and Ewing's sarcoma.
Teratoma (a germ cell tumor) often is cited as the most common tumor in this age group, but most teratomas are surgically removed while still benign, hence not necessarily cancer. Prior to the widespread routine use of prenatal ultrasound examinations, the incidence of sacrococcygeal teratomas diagnosed at birth was 25 to 29 per million births.
Female and male infants have essentially the same overall cancer incidence rates, a notable difference compared to older children.
White infants have higher cancer rates than black infants. Leukemias accounted for a substantial proportion of this difference: the average annual rate for white infants (48.7 per million) was 66% higher than for black infants (29.4 per million).[5]
Relative survival for infants is very good for neuroblastoma, Wilms' tumor and retinoblastoma, and fairly good (80%) for leukemia, but not for most other types of cancer.
Signs and symptoms
Roughly, cancer symptoms can be divided into three groups:
- Local symptoms: unusual lumps or swelling (tumor), hemorrhage (bleeding), pain and/or ulceration. Compression of surrounding tissues may cause symptoms such as jaundice (yellowing the eyes and skin).
- Symptoms of metastasis (spreading): enlarged lymph nodes, cough and hemoptysis, hepatomegaly (enlarged liver), bone pain, fracture of affected bones and neurological symptoms. Although advanced cancer may cause pain, it is often not the first symptom.
- Systemic symptoms: weight loss, poor appetite, fatigue and cachexia (wasting), excessive sweating (night sweats), anemia and specific paraneoplastic phenomena, i.e. specific conditions that are due to an active cancer, such as thrombosis or hormonal changes.
Every symptom in the above list can be caused by a variety of conditions (a list of which is referred to as the differential diagnosis). Cancer may be a common or uncommon cause of each item.
Diagnosis
Most cancers are initially recognized either because signs or symptoms appear or through screening. Neither of these lead to a definitive diagnosis, which usually requires the opinion of a pathologist, a type of physician (medical doctor) who specializes in the diagnosis of cancer and other diseases.
People with suspected cancer are investigated with medical tests. These commonly include blood tests, X-rays, CT scans and endoscopy.
Biopsy
A cancer may be suspected for a variety of reasons, but the definitive diagnosis of most malignancies must be confirmed by histological examination of the cancerous cells by a pathologist. Tissue can be obtained from a biopsy or surgery. Many biopsies (such as those of the skin, breast or liver) can be done in a doctor's office. Biopsies of other organs are performed under anesthesia and require surgery in an operating room.
The tissue diagnosis given by the pathologist indicates the type of cell that is proliferating, its histological grade and other features of the tumor. Together, this information is useful to evaluate the prognosis of this patient and to choose the best treatment. Cytogenetics and immunohistochemistry are other types of testing that the pathologist may perform on the tissue specimen. These tests may provide information about future behavior of the cancer (prognosis) and best treatment.
Treatment
Cancer can be treated by surgery, chemotherapy, radiation therapy, immunotherapy, monoclonal antibody therapy or other methods. The choice of therapy depends upon the location and grade of the tumor and the stage of the disease, as well as the general state of the patient (performance status). A number of experimental cancer treatments are also under development.
Complete removal of the cancer without damage to the rest of the body is the goal of treatment. Sometimes this can be accomplished by surgery, but the propensity of cancers to invade adjacent tissue or to spread to distant sites by microscopic metastasis often limits its effectiveness. The effectiveness of chemotherapy is often limited by toxicity to other tissues in the body. Radiation can also cause damage to normal tissue.
Because "cancer" refers to a class of diseases, it is unlikely that there will ever be a single "cure for cancer" any more than there will be a single treatment for all infectious diseases.
Surgery
In theory, non-hematological cancers can be cured if entirely removed by surgery, but this is not always possible. When the cancer has metastasized to other sites in the body prior to surgery, complete surgical excision is usually impossible. In the Halstedian model of cancer progression, tumors grow locally, then spread to the lymph nodes, then to the rest of the body. This has given rise to the popularity of local-only treatments such as surgery for small cancers. Even small localized tumors are increasingly recognized as possessing metastatic potential.
Examples of surgical procedures for cancer include mastectomy for breast cancer and prostatectomy for prostate cancer. The goal of the surgery can be either the removal of only the tumor, or the entire organ. A single cancer cell is invisible to the naked eye but can regrow into a new tumor, a process called recurrence. For this reason, the pathologist will examine the surgical specimen to determine if a margin of healthy tissue is present, thus decreasing the chance that microscopic cancer cells are left in the patient.
In addition to removal of the primary tumor, surgery is often necessary for staging, e.g. determining the extent of the disease and whether it has metastasized to regional lymph nodes. Staging is a major determinant of prognosis and of the need for adjuvant therapy.
Occasionally, surgery is necessary to control symptoms, such as spinal cord compression or bowel obstruction. This is referred to as palliative treatment.
Radiation therapy
Radiation therapy (also called radiotherapy, X-ray therapy, or irradiation) is the use of ionizing radiation to kill cancer cells and shrink tumors. Radiation therapy can be administered externally via external beam radiotherapy (EBRT) or internally via brachytherapy. The effects of radiation therapy are localised and confined to the region being treated. Radiation therapy injures or destroys cells in the area being treated (the "target tissue") by damaging their genetic material, making it impossible for these cells to continue to grow and divide. Although radiation damages both cancer cells and normal cells, most normal cells can recover from the effects of radiation and function properly. The goal of radiation therapy is to damage as many cancer cells as possible, while limiting harm to nearby healthy tissue. Hence, it is given in many fractions, allowing healthy tissue to recover between fractions.
Radiation therapy may be used to treat almost every type of solid tumor, including cancers of the brain, breast, cervix, larynx, lung, pancreas, prostate, skin, stomach, uterus, or soft tissue sarcomas. Radiation is also used to treat leukemia and lymphoma. Radiation dose to each site depends on a number of factors, including the radiosensitivity of each cancer type and whether there are tissues and organs nearby that may be damaged by radiation. Thus, as with every form of treatment, radiation therapy is not without its side effects.
Chemotherapy
Chemotherapy is the treatment of cancer with drugs ("anticancer drugs") that can destroy cancer cells. In current usage, the term "chemotherapy" usually refers to cytotoxic drugs which affect rapidly dividing cells in general, in contrast with targeted therapy (see below). Chemotherapy drugs interfere with cell division in various possible ways, e.g. with the duplication of DNA or the separation of newly formed chromosomes. Most forms of chemotherapy target all rapidly dividing cells and are not specific for cancer cells, although some degree of specificity may come from the inability of many cancer cells to repair DNA damage, while normal cells generally can. Hence, chemotherapy has the potential to harm healthy tissue, especially those tissues that have a high replacement rate (e.g. intestinal lining). These cells usually repair themselves after chemotherapy.
Because some drugs work better together than alone, two or more drugs are often given at the same time. This is called "combination chemotherapy"; most chemotherapy regimens are given in a combination.
The treatment of some leukaemias and lymphomas requires the use of high-dose chemotherapy, and total body irradiation (TBI). This treatment ablates the bone marrow, and hence the body's ability to recover and repopulate the blood. For this reason, bone marrow, or peripheral blood stem cell harvesting is carried out before the ablative part of the therapy, to enable "rescue" after the treatment has been given. This is known as autologous stem cell transplantation. Alternatively, hematopoietic stem cells may be transplanted from a matched unrelated donor (MUD).
Targeted therapies
Targeted therapy, which first became available in the late 1990s, has had a significant impact in the treatment of some types of cancer, and is currently a very active research area. This constitutes the use of agents specific for the deregulated proteins of cancer cells. Small molecule targeted therapy drugs are generally inhibitors of enzymatic domains on mutated, overexpressed, or otherwise critical proteins within the cancer cell. Prominent examples are the tyrosine kinase inhibitors imatinib and gefitinib.
Monoclonal antibody therapy is another strategy in which the therapeutic agent is an antibody which specifically binds to a protein on the surface of the cancer cells. Examples include the anti-HER2/neu antibody trastuzumab (Herceptin) used in breast cancer, and the anti-CD20 antibody rituximab, used in a variety of B-cell malignancies.
Targeted therapy can also involve small peptides as "homing devices" which can bind to cell surface receptors or affected extracellular matrix surrounding the tumor. Radionuclides which are attached to this peptides (e.g. RGDs) eventually kill the cancer cell if the nuclide decays in the vicinity of the cell. Especially oligo- or multimers of these binding motifs are of great interest, since this can lead to enhanced tumor specificity and avidity.
Photodynamic therapy (PDT) is a ternary treatment for cancer involving a photosensitizer, tissue oxygen, and light (often using lasers). PDT can be used as treatment for basal cell carcinoma (BCC) or lung cancer; PDT can also be useful in removing traces of malignant tissue after surgical removal of large tumors.[7]
Immunotherapy
Cancer immunotherapy refers to a diverse set of therapeutic strategies designed to induce the patient's own immune system to fight the tumor. Contemporary methods for generating an immune response against tumours include intravesical BCG immunotherapy for superficial bladder cancer, and use of interferons and other cytokines to induce an immune response in renal cell carcinoma and melanoma patients. Vaccines to generate specific immune responses are the subject of intensive research for a number of tumours, notably malignant melanoma and renal cell carcinoma. Sipuleucel-T is a vaccine-like strategy in late clinical trials for prostate cancer in which dendritic cells from the patient are loaded with prostatic acid phosphatase peptides to induce a specific immune response against prostate-derived cells.
Allogeneic hematopoietic stem cell transplantation ("bone marrow transplantation" from a genetically non-identical donor) can be considered a form of immunotherapy, since the donor's immune cells will often attack the tumor in a phenomenon known as graft-versus-tumor effect. For this reason, allogeneic HSCT leads to a higher cure rate than autologous transplantation for several cancer types, although the side effects are also more severe.
Hormonal therapy
The growth of some cancers can be inhibited by providing or blocking certain hormones. Common examples of hormone-sensitive tumors include certain types of breast and prostate cancers. Removing or blocking estrogen or testosterone is often an important additional treatment. In certain cancers, administration of hormone agonists, such as progestogens may be therapeutically beneficial.
Angiogenesis inhibitors
Angiogenesis inhibitors prevent the extensive growth of blood vessels (angiogenesis) that tumors require to survive. Some, such as bevacizumab, have been approved and are in clinical use. One of the main problems with anti-angiogenesis drugs is that many factors stimulate blood vessel growth, in normal cells and cancer. Anti-angiogenesis drugs only target one factor, so the other factors continue to stimulate blood vessel growth. Other problems include route of administration, maintenance of stability and activity and targeting at the tumor vasculature.[8]
Symptom control
Although the control of the symptoms of cancer is not typically thought of as a treatment directed at the cancer, it is an important determinant of the quality of life of cancer patients, and plays an important role in the decision whether the patient is able to undergo other treatments. Although doctors generally have the therapeutic skills to reduce pain, nausea, vomiting, diarrhea, hemorrhage and other common problems in cancer patients, the multidisciplinary specialty of palliative care has arisen specifically in response to the symptom control needs of this group of patients.
Pain medication, such as morphine and oxycodone, and antiemetics, drugs to suppress nausea and vomiting, are very commonly used in patients with cancer-related symptoms. Improved antiemetics such as ondansetron and analogues, as well as aprepitant have made aggressive treatments much more feasible in cancer patients.
Chronic pain due to cancer is almost always associated with continuing tissue damage due to the disease process or the treatment (i.e. surgery, radiation, chemotherapy). Although there is always a role for environmental factors and affective disturbances in the genesis of pain behaviors, these are not usually the predominant etiologic factors in patients with cancer pain. Furthermore, many patients with severe pain associated with cancer are nearing the end of their lives and palliative therapies are required. Issues such as social stigma of using opioids, work and functional status, and health care consumption are not likely to be important in the overall case management. Hence, the typical strategy for cancer pain management is to get the patient as comfortable as possible using opioids and other medications, surgery, and physical measures. Doctors have been reluctant to prescribe narcotics for pain in terminal cancer patients, for fear of contributing to addiction or suppressing respiratory function. The palliative care movement, a more recent offshoot of the hospice movement, has engendered more widespread support for preemptive pain treatment for cancer patients.
Fatigue is a very common problem for cancer patients, and has only recently become important enough for oncologists to suggest treatment, even though it plays a significant role in many patients' quality of life.
Treatment trials
Clinical trials, also called research studies, test new treatments in people with cancer. The goal of this research is to find better ways to treat cancer and help cancer patients. Clinical trials test many types of treatment such as new drugs, new approaches to surgery or radiation therapy, new combinations of treatments, or new methods such as gene therapy.
A clinical trial is one of the final stages of a long and careful cancer research process. The search for new treatments begins in the laboratory, where scientists first develop and test new ideas. If an approach seems promising, the next step may be testing a treatment in animals to see how it affects cancer in a living being and whether it has harmful effects. Of course, treatments that work well in the lab or in animals do not always work well in people. Studies are done with cancer patients to find out whether promising treatments are safe and effective.
Patients who take part may be helped personally by the treatment they receive. They get up-to-date care from cancer experts, and they receive either a new treatment being tested or the best available standard treatment for their cancer. At the same time, new treatments also may have unknown risks, but if a new treatment proves effective or more effective than standard treatment, study patients who receive it may be among the first to benefit. There is no guarantee that a new treatment being tested or a standard treatment will produce good results. In children with cancer, a survey of trials found that those enrolled in trials were on average not more likely to do better or worse than those on standard treatment; this confirms that success or failure of an experimental treatment cannot be predicted.[9]
Complementary and alternative
Complementary and alternative medicine (CAM) treatments are the diverse group of medical and health care systems, practices, and products that are not part of conventional medicine.[10] "Complementary medicine" refers to methods and substances used along with conventional medicine, while "alternative medicine" refers to compounds used instead of conventional medicine.[11] CAM use is common among people with cancer; a 2000 study found that 69% cancer patients had used at least one CAM therapy as part of their cancer treatment.[12] Most complementary and alternative medicines for cancer have not been rigorously studied or tested. Some alternative treatments which have been investigated and shown to be ineffective continue to be marketed and promoted.[13]
Prognosis
Cancer has a reputation for being a deadly disease. While this certainly applies to certain particular types, the truths behind the historical connotations of cancer are increasingly being overturned by advances in medical care. Some types of cancer have a prognosis that is substantially better than nonmalignant diseases such as heart failure and stroke.
Progressive and disseminated malignant disease has a substantial impact on a cancer patient's quality of life, and many cancer treatments (such as chemotherapy) may have severe side-effects. In the advanced stages of cancer, many patients need extensive care, affecting family members and friends. Palliative care solutions may include permanent or "respite" hospice nursing.
Emotional impact
Many local organizations offer a variety of practical and support services to people with cancer. Support can take the form of support groups, counseling, advice, financial assistance, transportation to and from treatment, films or information about cancer. Neighborhood organizations, local health care providers, or area hospitals may have resources or services available.
Counseling can provide emotional support to cancer patients and help them better understand their illness. Different types of counseling include individual, group, family, peer counseling, bereavement, patient-to-patient, and sexuality.
Many governmental and charitable organizations have been established to help patients cope with cancer. These organizations often are involved in cancer prevention, cancer treatment, and cancer research.
Causes
Cancer is a diverse class of diseases which differ widely in their causes and biology. The common thread in all known cancers is the acquisition of abnormalities in the genetic material of the cancer cell and its progeny. Research into the pathogenesis of cancer can be divided into three broad areas of focus. The first area of research focuses on the agents and events which cause or facilitate genetic changes in cells destined to become cancer. Second, it is important to uncover the precise nature of the genetic damage, and the genes which are affected by it. The third focus is on the consequences of those genetic changes on the biology of the cell, both in generating the defining properties of a cancer cell, and in facilitating additional genetic events, leading to further progression of the cancer.
Mutation: chemical carcinogens
Cancer pathogenesis is traceable back to DNA mutations that impact cell growth and metastasis. Substances that cause DNA mutations are known as mutagens, and mutagens that cause cancers are known as carcinogens. Particular substances have been linked to specific types of cancer. Tobacco smoking is associated with many forms of cancer,[14] and causes 90% of lung cancer.[15] Prolonged exposure to asbestos fibers is associated with mesothelioma.[16]
Many mutagens are also carcinogens, but some carcinogens are not mutagens. Alcohol is an example of a chemical carcinogen that is not a mutagen.[17] Such chemicals may promote cancers through stimulating the rate of cell division. Faster rates of replication leaves less time for repair enzymes to repair damaged DNA during DNA replication, increasing the likelihood of a mutation.
Decades of research has demonstrated the link between tobacco use and cancer in the lung, larynx, head, neck, stomach, bladder, kidney, oesophagus and pancreas.[18] Tobacco smoke contains over fifty known carcinogens, including nitrosamines and polycyclic aromatic hydrocarbons.[19] Tobacco is responsible for about one in three of all cancer deaths in the developed world,[14] and about one in five worldwide.[19] Indeed, lung cancer death rates in the United States have mirrored smoking patterns, with increases in smoking followed by dramatic increases in lung cancer death rates and, more recently, decreases in smoking followed by decreases in lung cancer death rates in men. However, the numbers of smokers worldwide is still rising, leading to what some organizations have described as the tobacco epidemic.[20]
Mutation: ionizing radiation
Sources of ionizing radiation, such as radon gas, can cause cancer. Prolonged exposure to ultraviolet radiation from the sun can lead to melanoma and other skin malignancies.[21]
Radio-frequency radiation from mobile phones has been proposed as a cause of cancer, but there is little evidence of such a link.[22] Nevertheless, some experts caution against prolonged exposure.[23]
Viral or bacterial infection
Some cancers can be caused by infection with pathogens.[24] Many cancers originate from a viral infection; this is especially true in animals such as birds, but also in humans, as viruses are responsible for 15% of human cancers worldwide. The main viruses associated with human cancers are human papillomavirus, hepatitis B and hepatitis C virus, Epstein-Barr virus, and human T-lymphotropic virus. Experimental and epidemiological data imply a causative role for viruses and they appear to be the second most important risk factor for cancer development in humans, exceeded only by tobacco usage.[25] The mode of virally-induced tumors can be divided into two, acutely-transforming or slowly-transforming. In acutely transforming viruses, the virus carries an overactive oncogene called viral-oncogene (v-onc), and the infected cell is transformed as soon as v-onc is expressed. In contrast, in slowly-transforming viruses, the virus genome is inserts near a proto-oncogene in the host genome. The viral promoter or other transcription regulation elements then cause overexpression of that proto-oncogene. This induces uncontrolled cell division. Because the site of insertion is not specific to proto-oncogenes and the chance of insertion near any proto-oncogene is low, slowly-transforming viruses will cause tumors much longer after infection than the acutely-transforming viruses.
Hepatitis viruses, including hepatitis B and hepatitis C, can induce a chronic viral infection that leads to liver cancer in 0.47% of hepatitis B patients per year (especially in Asia, less so in North America), and in 1.4% of hepatitis C carriers per year. Liver cirrhosis, whether from chronic viral hepatitis infection or alcoholism, is associated with the development of liver cancer, and the combination of cirrhosis and viral hepatitis presents the highest risk of liver cancer development. Worldwide, liver cancer is one of the most common, and most deadly, cancers due to a huge burden of viral hepatitis transmission and disease.
Advances in cancer research have made a vaccine designed to prevent cancer available. In 2006, the US FDA approved a human papilloma virus vaccine, called Gardasil. The vaccine protects against four HPV types, which together cause 70% of cervical cancers and 90% of genital warts. In March 2007, the US CDC Advisory Committee on Immunization Practices (ACIP) officially recommended that females aged 11-12 receive the vaccine, and indicated that females as young as age 9 and as old as age 26 are also candidates for immunization.
In addition to viruses, researchers have noted a connection between bacteria and certain cancers. The most prominent example is the link between chronic infection of the wall of the stomach with Helicobacter pylori and gastric cancer.[26][27] Although only a minority of those infected with Helicobacter go on to develop cancer, since this pathogen is quite common it is probably responsible for the majority of these cancers.[28]
Hormonal imbalances
Some hormones can act in a similar manner to non-mutagenic carcinogens in that they may stimulate excessive cell growth. A well-established example is the role of hyperestrogenic states in promoting endometrial cancer.
Immune system dysfunction
HIV is associated with a number of malignancies, including Kaposi's sarcoma, non-Hodgkin's lymphoma, and HPV-associated malignancies such as anal cancer and cervical cancer. AIDS-defining illnesses have long included these diagnoses. The increased incidence of malignancies in HIV patients points to the breakdown of immune surveillance as a possible etiology of cancer.[29] Certain other immune deficiency states (e.g. common variable immunodeficiency and IgA deficiency) are also associated with increased risk of malignancy.[30]
Heredity
Most forms of cancer are "sporadic", and have no basis in heredity. There are, however, a number of recognised syndromes of cancer with a hereditary component, often a defective tumor suppressor allele. Famous examples are:
- certain inherited mutations in the genes BRCA1 and BRCA2 are associated with an elevated risk of breast cancer and ovarian cancer
- tumors of various endocrine organs in multiple endocrine neoplasia (MEN types 1, 2a, 2b)
- Li-Fraumeni syndrome (various tumors such as osteosarcoma, breast cancer, soft tissue sarcoma, brain tumors) due to mutations of p53
- Turcot syndrome (brain tumors and colonic polyposis)
- Familial adenomatous polyposis an inherited mutation of the APC gene that leads to early onset of colon carcinoma.
- Hereditary nonpolyposis colorectal cancer (HNPCC, also known as Lynch syndrome) can include familial cases of colon cancer, uterine cancer, gastric cancer, and ovarian cancer, without a preponderance of colon polyps.
- Retinoblastoma, when occurring in young children, is due to a hereditary mutation in the retinoblastoma gene.
- Down syndrome patients, who have an extra chromosome 21, are known to develop malignancies such as leukemia and testicular cancer, though the reasons for this difference are not well understood.
Other causes
A few types of cancer in non-humans have been found to be caused by the tumor cells themselves. This phenomenon is seen in dogs with Sticker's sarcoma, also known as canine transmissible venereal tumor[31], as well as Devil facial tumour disease in Tasmanian devils. The closest known analogue to this in humans is individuals who have developed cancer from transplacental transmission or from organ transplants.
In the United States every year, approximately 3,500 pregnant women have a malignancy, and transplacental transmission of acute leukaemia, lymphoma, melanoma and carcinoma from mother to fetus has been observed. [32]
The development of donor-derived tumors from organ transplants is exceedingly rare. The main cause of organ transplant associated tumors seems to be malignant melanoma, that was undetected at the time of organ harvest. [33]
Pathophysiology
Cancer is fundamentally a disease of regulation of tissue growth. In order for a normal cell to transform into a cancer cell, genes which regulate cell growth and differentiation must be altered.[34] Genetic changes can occur at many levels, from gain or loss of entire chromosomes to a mutation affecting a single DNA nucleotide. There are two broad categories of genes which are affected by these changes. Oncogenes may be normal genes which are expressed at inappropriately high levels, or altered genes which have novel properties. In either case, expression of these genes promotes the malignant phenotype of cancer cells. Tumor suppressor genes are genes which inhibit cell division, survival, or other properties of cancer cells. Tumor suppressor genes are often disabled by cancer-promoting genetic changes. Typically, changes in many genes are required to transform a normal cell into a cancer cell.[35]
There is a diverse classification scheme for the various genomic changes which may contribute to the generation of cancer cells. Most of these changes are mutations, or changes in the nucleotide sequence of genomic DNA. Aneuploidy, the presence of an abnormal number of chromosomes, is one genomic change which is not a mutation, and may involve either gain or loss of one or more chromosomes through errors in mitosis.
Large-scale mutations involve the deletion or gain of a portion of a chromosome. Genomic amplification occurs when a cell gains many copies (often 20 or more) of a small chromosomal locus, usually containing one or more oncogenes and adjacent genetic material. Translocation occurs when two separate chromosomal regions become abnormally fused, often at a characteristic location. A well-known example of this is the Philadelphia chromosome, or translocation of chromosomes 9 and 22, which occurs in chronic myelogenous leukemia, and results in production of the BCR-abl fusion protein, an oncogenic tyrosine kinase.
Small-scale mutations include point mutations, deletions, and insertions, which may occur in the promoter of a gene and affect its expression, or may occur in the gene's coding sequence and alter the function or stability of its protein product. Disruption of a single gene may also result from integration of genomic material from a DNA virus or retrovirus, and such an event may also result in the expression of viral oncogenes in the affected cell and its descendants.
Epigenetics
Epigenetics is the study of the regulation of gene expression through chemical, non-mutational changes in DNA structure. The theory of epigenetics in cancer pathogenesis is that non-mutational changes to DNA can lead to alterations in gene expression. Normally, oncogenes are silent, for example, because of DNA methylation. Loss of that methylation can induce the aberrant expression of oncogenes, leading to cancer pathogenesis. Known mechanisms of epigenetic change include DNA methylation, and methylation or acetylation of histone proteins bound to chromosomal DNA at specific locations. Classes of medications, known as HDAC inhibitors and DNA methyltransferase inhibitors, can re-regulate the epigenetic signaling in the cancer cell.
Oncogenes
Oncogenes promote cell growth through a variety of ways. Many can produce hormones, a "chemical messenger" between cells which encourage mitosis, the effect of which depends on the signal transduction of the receiving tissue or cells. In other words, when a hormone receptor on a recipient cell is stimulated, the signal is conducted from the surface of the cell to the cell nucleus to effect some change in gene transcription regulation at the nuclear level. Some oncogenes are part of the signal transduction system itself, or the signal receptors in cells and tissues themselves, thus controlling the sensitivity to such hormones. Oncogenes often produce mitogens, or are involved in transcription of DNA in protein synthesis, which creates the proteins and enzymes responsible for producing the products and biochemicals cells use and interact with.
Mutations in proto-oncogenes, which are the normally quiescent counterparts of oncogenes, can modify their expression and function, increasing the amount or activity of the product protein. When this happens, the proto-oncogenes become oncogenes, and this transition upsets the normal balance of cell cycle regulation in the cell, making uncontrolled growth possible. The chance of cancer cannot be reduced by removing proto-oncogenes from the genome, even if this were possible, as they are critical for growth, repair and homeostasis of the organism. It is only when they become mutated that the signals for growth become excessive.
One of the first oncogenes to be defined in cancer research is the ras oncogene. Mutations in the Ras family of proto-oncogenes (comprising H-Ras, N-Ras and K-Ras) are very common, being found in 20% to 30% of all human tumours.[36] Ras was originally identified in the Harvey sarcoma virus genome, and researchers were surprised that not only was this gene present in the human genome but that, when ligated to a stimulating control element, could induce cancers in cell line cultures.[37]
Tumor suppressor genes
Tumor suppressor genes code for anti-proliferation signals and proteins that suppress mitosis and cell growth. Generally, tumor suppressors are transcription factors that are activated by cellular stress or DNA damage. Often DNA damage will cause the presence of free-floating genetic material as well as other signs, and will trigger enzymes and pathways which lead to the activation of tumor suppressor genes. The functions of such genes is to arrest the progression of the cell cycle in order to carry out DNA repair, preventing mutations from being passed on to daughter cells. The p53 protein, one of the most important studied tumor suppressor genes, is a transcription factor activated by many cellular stressors including hypoxia and ultraviolet radiation damage.
Despite nearly half of all cancers possibly involving alterations in p53, its tumor suppressor function is poorly understood. p53 clearly has two functions: one a nuclear role as a transcription factor, and the other a cytoplasmic role in regulating the cell cycle, cell division, and apoptosis.
The Warburg hypothesis is the preferential use of glycolysis for energy to sustain cancer growth. p53 has been shown to regulate the shift from the respiratory to the glycolytic pathway.[38]
However, a mutation can damage the tumor suppressor gene itself, or the signal pathway which activates it, "switching it off". The invariable consequence of this is that DNA repair is hindered or inhibited: DNA damage accumulates without repair, inevitably leading to cancer.
Mutations of tumor suppressor genes that occur in germline cells are passed along to offspring, and increase the likelihood for cancer diagnoses in subsequent generations. Members of these families have increased incidence and decreased latency of multiple tumors. The tumor types are typical for each type of tumor suppressor gene mutation, with some mutations causing particular cancers, and other mutations causing others. The mode of inheritance of mutant tumor suppressors is that an affected member inherits a defective copy from one parent, and a normal copy from the other. For instance, individuals who inherit one mutant p53 allele (and are therefore heterozygous for mutated p53) can develop melanomas and pancreatic cancer, known as Li-Fraumeni syndrome. Other inherited tumor suppressor gene syndromes include Rb mutations, linked to retinoblastoma, and APC gene mutations, linked to adenopolyposis colon cancer. Adenopolyposis colon cancer is associated with thousands of polyps in colon while young, leading to colon cancer at a relatively early age. Finally, inherited mutations in BRCA1 and BRCA2 lead to early onset of breast cancer.
Development of cancer was proposed in 1971 to depend on at least two mutational events. In what became known as the Knudson two-hit hypothesis, an inherited, germ-line mutation in a tumor suppressor gene would only cause cancer if another mutation event occurred later in the organism's life, inactivating the other allele of that tumor suppressor gene.[39]
Usually, oncogenes are dominant, as they contain gain-of-function mutations, while mutated tumor suppressors are recessive, as they contain loss-of-function mutations. Each cell has two copies of the same gene, one from each parent, and under most cases gain of function mutations in just one copy of a particular proto-oncogene is enough to make that gene a true oncogene. On the other hand, loss of function mutations need to happen in both copies of a tumor suppressor gene to render that gene completely non-functional. However, cases exist in which one mutated copy of a tumor suppressor gene can render the other, wild-type copy non-functional. This phenomenon is called the dominant negative effect and is observed in many p53 mutations.
Knudson’s two hit model has recently been challenged by several investigators. Inactivation of one allele of some tumor suppressor genes is sufficient to cause tumors. This phenomenon is called haploinsufficiency and has been demonstrated by a number of experimental approaches. Tumors caused by haploinsufficiency usually have a later age of onset when compared with those by a two hit process.[40]
Often, the multiple genetic changes which result in cancer may take many years to accumulate. During this time, the biological behavior of the pre-malignant cells slowly change from the properties of normal cells to cancer-like properties. Pre-malignant tissue can have a distinctive appearance under the microscope. Among the distinguishing traits are an increased number of dividing cells, variation in nuclear size and shape, variation in cell size and shape, loss of specialized cell features, and loss of normal tissue organization. Dysplasia is an abnormal type of excessive cell proliferation characterized by loss of normal tissue arrangement and cell structure in pre-malignant cells. These early neoplastic changes must be distinguished from hyperplasia, a reversible increase in cell division caused by an external stimulus, such as a hormonal imbalance or chronic irritation.
The most severe cases of dysplasia are referred to as "carcinoma in situ." In Latin, the term "in situ" means "in place", so carcinoma in situ refers to an uncontrolled growth of cells that remains in the original location and has not shown invasion into other tissues. Nevertheless, carcinoma in situ may develop into an invasive malignancy and is usually removed surgically, if possible.
Clonal evolution
Main article: Somatic evolution in cancer
The process by which normal tissue becomes malignant is a process of somatic evolution within the body[41]. Millions of years of biological evolution insure that the cellular metabolic changes that enable cancer to grow occur only very rarely. Most changes in cellular metabolism that allow cells to grow in a disorderly fashion lead to cell death. Cancer cells undergo a process of natural selection, in that the few cells with new genetic changes that enhance their survival or reproduction continue to multiply, and soon come to dominate the growing tumor, as cells with less favorable genetic change are out-competed[42]. This process is called clonal evolution. Tumors often continue to evolve in response to chemotherapy treatments, and on occasion aberrant cells may acquire resistance to particular anti-cancer pharmaceuticals.
Biological properties of cancer cells
In a 2000 article by Hanahan and Weinberg, the biological properties of malignant tumor cells were summarized as follows:[43]
- Acquisition of self-sufficiency in growth signals, leading to unchecked growth.
- Loss of sensitivity to anti-growth signals, also leading to unchecked growth.
- Loss of capacity for apoptosis, in order to allow growth despite genetic errors and external anti-growth signals.
- Loss of capacity for senescence, leading to limitless replicative potential (immortality)
- Acquisition of sustained angiogenesis, allowing the tumor to grow beyond the limitations of passive nutrient diffusion.
- Acquisition of ability to invade neighbouring tissues, the defining property of invasive carcinoma.
- Acquisition of ability to build metastases at distant sites, the classical property of malignant tumors (carcinomas or others).
The completion of these multiple steps would be a very rare event without :
- Loss of capacity to repair genetic errors, leading to an increased mutation rate (genomic instability), thus accelerating all the other changes.
These biological changes are classical in carcinomas; other malignant tumor may not need all to achieve them all. For example, tissue invasion and displacement to distant sites are normal properties of leukocytes; these steps are not needed in the development of leukemia. The different steps do not necessarily represent individual mutations. For example, inactivation of a single gene, coding for the p53 protein, will cause genomic instability, evasion of apoptosis and increased angiogenesis. Not all the cancer cells are dividing. Rather, a subset of the cells in a tumor, called cancer stem cells, replicate themselves and generate differentiated cells.[44]
Prevention
Cancer prevention is defined as active measures to decrease the incidence of cancer. This can be accomplished by avoiding carcinogens or altering their metabolism, pursuing a lifestyle or diet that modifies cancer-causing factors and/or medical intervention (chemoprevention, treatment of pre-malignant lesions). The epidemiological concept of "prevention" is usually defined as either primary prevention, for people who have not been diagnosed with a particular disease, or secondary prevention, aimed at reducing recurrence or complications of a previously diagnosed illness.
Observational epidemiological studies that show associations between risk factors and specific cancers mostly serve to generate hypotheses about potential interventions that could reduce cancer incidence or morbidity. Randomized controlled trials then test whether hypotheses generated by epidemiological trials and laboratory research actually result in reduced cancer incidence and mortality. In many cases, findings from observational epidemiological studies are not confirmed by randomized controlled trials.
About a third of the twelve most common cancers worldwide are due to nine potentially modifiable risk factors. Men with cancer are twice as likely as women to have a modifiable risk factor for their disease. The nine risk factors are tobacco smoking, excessive alcohol use, diet low in fruit and vegetables, limited physical exercise, human papillomavirus infection (unsafe sex), urban air pollution, domestic use of solid fuels, and contaminated injections (hepatitis B and C).[45]
Modifiable ("lifestyle") risk factors
The vast majority of cancer risk factors are environmental or lifestyle-related in nature, leading to the claim that cancer is a largely preventable disease.[45] Examples of modifiable cancer risk factors include alcohol consumption (associated with increased risk of oral, esophageal, breast, and other cancers), smoking (although 20% of women with lung cancer have never smoked, versus 10% of men[46]), physical inactivity (associated with increased risk of colon, breast, and possibly other cancers), and being overweight (associated with colon, breast, endometrial, and possibly other cancers). Based on epidemiologic evidence, it is now thought that avoiding excessive alcohol consumption may contribute to reductions in risk of certain cancers; however, compared with tobacco exposure, the magnitude of effect is modest or small and the strength of evidence is often weaker. Other lifestyle and environmental factors known to affect cancer risk (either beneficially or detrimentally) include certain sexually transmitted diseases (such as those conveyed by the human papillomavirus), the use of exogenous hormones, exposure to ionizing radiation and ultraviolet radiation, and certain occupational and chemical exposures.
Every year, at least 200,000 people die worldwide from cancer related to their workplace.[47] Millions of workers run the risk of developing cancers such as lung cancer and mesothelioma from inhaling asbestos fibers and tobacco smoke, or leukemia from exposure to benzene at their workplaces.[47] Currently, most cancer deaths caused by occupational risk factors occur in the developed world.[47] It is estimated that approximately 20,000 cancer deaths and 40,000 new cases of cancer each year in the U.S. are attributable to occupation.[48]
See alcohol and cancer for more on that topic.
Diet
The consensus on diet and cancer is that obesity increases the risk of developing cancer. Particular dietary practices often explain differences in cancer incidence in different countries (e.g. gastric cancer is more common in Japan, while colon cancer is more common in the United States). Studies have shown that immigrants develop the risk of their new country, often within one generation, suggesting a substantial link between diet and cancer.[49] Whether reducing obesity in a population also reduces cancer incidence is unknown.
Despite frequent reports of particular substances (including foods) having a beneficial or detrimental effect on cancer risk, few of these have an established link to cancer. These reports are often based on studies in cultured cell media or animals. Public health recommendations cannot be made on the basis of these studies until they have been validated in an observational (or occasionally a prospective interventional) trial in humans.
Proposed dietary interventions for primary cancer risk reduction generally gain support from epidemiological association studies. Examples of such studies include reports that reduced meat consumption is associated with decreased risk of colon cancer,[50] and reports that consumption of coffee is associated with a reduced risk of liver cancer.[51] Studies have linked consumption of grilled meat to an increased risk of stomach cancer,[52] colon cancer,[53] breast cancer,[54] and pancreatic cancer,[55] a phenomenon which could be due to the presence of carcinogens such as benzopyrene in foods cooked at high temperatures.
A 2005 secondary prevention study showed that consumption of a plant-based diet and lifestyle changes resulted in a reduction in cancer markers in a group of men with prostate cancer who were using no conventional treatments at the time.[56] These results were amplified by a 2006 study in which over 2,400 women were studied, half randomly assigned to a normal diet, the other half assigned to a diet containing less than 20% calories from fat. The women on the low fat diet were found to have a markedly lower risk of breast cancer recurrence, in the interim report of December, 2006.[57]
Recent studies have also demonstrated potential links between some forms of cancer and high consumption of refined sugars and other simple carbohydrates.[58][59][60][61][62] Although the degree of correlation and the degree of causality is still debated,[63][64][65] some organizations have in fact begun to recommend reducing intake of refined sugars and starches as part of their cancer prevention regimens.[66][67][68]
In November 2007, the American Institute for Cancer Research (AICR), in conjunction with the World Cancer Research Fund (WCRF), published Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective', "the most current and comprehensive analysis of the literature on diet, physical activity and cancer".[69] The WCRF/AICR Expert Report lists 10 recommendations that people can follow to help reduce their risk of developing cancer, including the following dietary guidelines: (1) reducing intake of foods and drinks that promote weight gain, namely energy-dense foods and sugary drinks, (2) eating mostly foods of plant origin, (3) limiting intake of red meat and avoiding processed meat, (4) limiting consumption of alcoholic beverages, and (5) reducing intake of salt and avoiding mouldy cereals (grains) or pulses (legumes).[70][71]
Vitamins
The idea that cancer can be prevented through vitamin supplementation stems from early observations correlating human disease with vitamin deficiency, such as pernicious anemia with vitamin B12 deficiency, and scurvy with Vitamin C deficiency. This has largely not been proven to be the case with cancer, and vitamin supplementation is largely not proving effective in preventing cancer. The cancer-fighting components of food are also proving to be more numerous and varied than previously understood, so patients are increasingly being advised to consume fresh, unprocessed fruits and vegetables for maximal health benefits.[72]
Epidemiological studies have shown that low vitamin D status is correlated to increased cancer risk.[73][74] However, the results of such studies need to be treated with caution, as they cannot show whether a correlation between two factors means that one causes the other (i.e. correlation does not imply causation).[75] The possibility that Vitamin D might protect against cancer has been contrasted with the risk of malignancy from sun exposure. Since exposure to the sun enhances natural human production of vitamin D, some cancer researchers have argued that the potential deleterious malignant effects of sun exposure are far outweighed by the cancer-preventing effects of extra vitamin D synthesis in sun-exposed skin. In 2002, Dr. William B. Grant claimed that 23,800 premature cancer deaths occur in the US annually due to insufficient UVB exposure (apparently via vitamin D deficiency).[76] This is higher than 8,800 deaths occurred from melanoma or squamous cell carcinoma, so the overall effect of sun exposure might be beneficial. Another research group[77][78] estimates that 50,000–63,000 individuals in the United States and 19,000 - 25,000 in the UK die prematurely from cancer annually due to insufficient vitamin D.
The case of beta-carotene provides an example of the importance of randomized clinical trials. Epidemiologists studying both diet and serum levels observed that high levels of beta-carotene, a precursor to vitamin A, were associated with a protective effect, reducing the risk of cancer. This effect was particularly strong in lung cancer. This hypothesis led to a series of large randomized clinical trials conducted in both Finland and the United States (CARET study) during the 1980s and 1990s. This study provided about 80,000 smokers or former smokers with daily supplements of beta-carotene or placebos. Contrary to expectation, these tests found no benefit of beta-carotene supplementation in reducing lung cancer incidence and mortality. In fact, the risk of lung cancer was slightly, but not significantly, increased by beta-carotene, leading to an early termination of the study.[79]
Results reported in the Journal of the American Medical Association (JAMA) in 2007 indicate that folic acid supplementation is not effective in preventing colon cancer, and folate consumers may be more likely to form colon polyps.[80]
Chemoprevention
The concept that medications could be used to prevent cancer is an attractive one, and many high-quality clinical trials support the use of such chemoprevention in defined circumstances.
Daily use of tamoxifen, a selective estrogen receptor modulator (SERM), typically for 5 years, has been demonstrated to reduce the risk of developing breast cancer in high-risk women by about 50%. A recent study reported that the selective estrogen receptor modulator raloxifene has similar benefits to tamoxifen in preventing breast cancer in high-risk women, with a more favorable side effect profile.[81]
Raloxifene is a SERM like tamoxifen; it has been shown (in the STAR trial) to reduce the risk of breast cancer in high-risk women equally as well as tamoxifen. In this trial, which studied almost 20,000 women, raloxifene had fewer side effects than tamoxifen, though it did permit more DCIS to form.[81]
Finasteride, a 5-alpha-reductase inhibitor, has been shown to lower the risk of prostate cancer, though it seems to mostly prevent low-grade tumors.[82] The effect of COX-2 inhibitors such as rofecoxib and celecoxib upon the risk of colon polyps have been studied in familial adenomatous polyposis patients[83] and in the general population.[84][85] In both groups, there were significant reductions in colon polyp incidence, but this came at the price of increased cardiovascular toxicity.
Genetic testing
Genetic testing for high-risk individuals is already available for certain cancer-related genetic mutations. Carriers of genetic mutations that increase risk for cancer incidence can undergo enhanced surveillance, chemoprevention, or risk-reducing surgery. Early identification of inherited genetic risk for cancer, along with cancer-preventing interventions such as surgery or enhanced surveillance, can be lifesaving for high-risk individuals.
| Gene | Cancer types | Availability |
| Breast, ovarian, pancreatic | Commercially available for clinical specimens | |
| Colon, uterine, small bowel, stomach, urinary tract | Commercially available for clinical specimens |
Vaccination
Vaccines to prevent infection by oncogenic infectious agents, as well as to mount an immune response against cancer-specific epitopes) and to potential venues for gene therapy for individuals with genetic mutations or polymorphisms that put them at high risk of cancer, are underway.
As reported above, a preventive human papillomavirus vaccine exists that targets certain sexually transmitted strains of human papillomavirus that are associated with the development of cervical cancer and genital warts. The only two HPV vaccines on the market as of October 2007 are Gardasil and Cervarix.
Screening
Cancer screening is an attempt to detect unsuspected cancers in an asymptomatic population. Screening tests suitable for large numbers of healthy people must be relatively affordable, safe, noninvasive procedures with acceptably low rates of false positive results. If signs of cancer are detected, more definitive and invasive follow up tests are performed to confirm the diagnosis.
Screening for cancer can lead to earlier diagnosis in specific cases. Early diagnosis may lead to extended life, but may also falsely prolong the lead time to death through lead time bias or length time bias.
A number of different screening tests have been developed for different malignancies. Breast cancer screening can be done by breast self-examination, though this approach was discredited by a 2005 study in over 300,000 Chinese women. Screening for breast cancer with mammograms has been shown to reduce the average stage of diagnosis of breast cancer in a population. Stage of diagnosis in a country has been shown to decrease within ten years of introduction of mammographic screening programs. Colorectal cancer can be detected through fecal occult blood testing and colonoscopy, which reduces both colon cancer incidence and mortality, presumably through the detection and removal of pre-malignant polyps. Similarly, cervical cytology testing (using the Pap smear) leads to the identification and excision of precancerous lesions. Over time, such testing has been followed by a dramatic reduction of cervical cancer incidence and mortality. Testicular self-examination is recommended for men beginning at the age of 15 years to detect testicular cancer. Prostate cancer can be screened using a digital rectal exam along with prostate specific antigen (PSA) blood testing, though some authorities (such as the US Preventive Services Task Force) recommend against routinely screening all men.
Screening for cancer is controversial in cases when it is not yet known if the test actually saves lives. The controversy arises when it is not clear if the benefits of screening outweigh the risks of follow-up diagnostic tests and cancer treatments. For example: when screening for prostate cancer, the PSA test may detect small cancers that would never become life threatening, but once detected will lead to treatment. This situation, called overdiagnosis, puts men at risk for complications from unnecessary treatment such as surgery or radiation. Follow up procedures used to diagnose prostate cancer (prostate biopsy) may cause side effects, including bleeding and infection. Prostate cancer treatment may cause incontinence (inability to control urine flow) and erectile dysfunction (erections inadequate for intercourse). Similarly, for breast cancer, there have recently been criticisms that breast screening programs in some countries cause more problems than they solve. This is because screening of women in the general population will result in a large number of women with false positive results which require extensive follow-up investigations to exclude cancer, leading to having a high number-to-treat (or number-to-screen) to prevent or catch a single case of breast cancer early.
Cervical cancer screening via the Pap smear has the best cost-benefit profile of all the forms of cancer screening from a public health perspective as, being largely caused by a virus, it has clear risk factors (sexual contact), and the natural progression of cervical cancer is that it normally spreads slowly over a number of years therefore giving more time for the screening program to catch it early. Moreover, the test itself is easy to perform and relatively cheap.
For these reasons, it is important that the benefits and risks of diagnostic procedures and treatment be taken into account when considering whether to undertake cancer screening.
Use of medical imaging to search for cancer in people without clear symptoms is similarly marred with problems. There is a significant risk of detection of what has been recently called an incidentaloma - a benign lesion that may be interpreted as a malignancy and be subjected to potentially dangerous investigations. Recent studies of CT scan-based screening for lung cancer in smokers have had equivocal results, and systematic screening is not recommended as of July 2007. Randomized clinical trials of plain-film chest X-rays to screen for lung cancer in smokers have shown no benefit for this approach.
Canine cancer detection has shown promise, but is still in the early stages of research.
Epidemiology
Cancer epidemiology is the study of the incidence of cancer as a way to infer possible trends and causes. The first such cause of cancer was identified by British surgeon Percivall Pott, who discovered in 1775 that cancer of the scrotum was a common disease among chimney sweeps. The work of other individual physicians led to various insights, but when physicians started working together they could make firmer conclusions.
A founding paper of this discipline was the work of Janet Lane-Claypon, who published a comparative study in 1926 of 500 breast cancer cases and 500 control patients of the same background and lifestyle for the British Ministry of Health. Her ground-breaking work on cancer epidemiology was carried on by Richard Doll and Austin Bradford Hill, who published "Lung Cancer and Other Causes of Death In Relation to Smoking. A Second Report on the Mortality of British Doctors" followed in 1956 (otherwise known as the British doctors study). Richard Doll left the London Medical Research Center (MRC), to start the Oxford unit for Cancer epidemiology in 1968. With the use of computers, the unit was the first to compile large amounts of cancer data. Modern epidemiological methods are closely linked to current concepts of disease and public health policy. Over the past 50 years, great efforts have been spent on gathering data across medical practise, hospital, provincial, state, and even country boundaries, as a way to study the interdependence of environmental and cultural factors on cancer incidence.
Cancer epidemiology must contend with problems of lead time bias and length time bias. Lead time bias is the concept that early diagnosis may artificially inflate the survival statistics of a cancer, without really improving the natural history of the disease. Length bias is the concept that slower growing, more indolent tumors are more likely to be diagnosed by screening tests, but improvements in diagnosing more cases of indolent cancer may not translate into better patient outcomes after the implementation of screening programs. A similar epidemiological concern is overdiagnosis, the tendency of screening tests to diagnose diseases that may not actually impact the patient's longevity. This problem especially applies to prostate cancer and PSA screening.[86]
Some cancer researchers have argued that negative cancer clinical trials lack sufficient statistical power to discover a benefit to treatment. This may be due to fewer patients enrolled in the study than originally planned.[87]
State and regional cancer registries are organizations that abstract clinical data about cancer from patient medical records. These institutions provide information to state and national public health groups to help track trends in cancer diagnosis and treatment. One of the largest and most important cancer registries is SEER, administered by the US Federal government.[88] Health information privacy concerns have led to the restricted use of cancer registry data in the United States Department of Veterans Affairs[89][90][91] and other institutions.[92]
In some Western countries, such as the USA,[4] and the UK[93] cancer is overtaking cardiovascular disease as the leading cause of death. In many Third World countries cancer incidence (insofar as this can be measured) appears much lower, most likely because of the higher death rates due to infectious disease or injury. With the increased control over malaria and tuberculosis in some Third World countries, incidence of cancer is expected to rise; this is termed the epidemiologic transition in epidemiological terminology.
Cancer epidemiology closely mirrors risk factor spread in various countries. Hepatocellular carcinoma (liver cancer) is rare in the West but is the main cancer in China and neighbouring countries, most likely due to the endemic presence of hepatitis B and aflatoxin in that population. Similarly, with tobacco smoking becoming more common in various Third World countries, lung cancer incidence has increased in a parallel fashion.
History
Today, the Greek term carcinoma is the medical term for a malignant tumor derived from epithelial cells. It is Celsus who translated carcinos into the Latin cancer, also meaning crab. Galen used "oncos" to describe all tumours, the root for the modern word oncology.[94]
Hippocrates described several kinds of cancers. He called benign tumours oncos, Greek for swelling, and malignant tumours carcinos, Greek for crab or crayfish. This name comes from the appearance of the cut surface of a solid malignant tumour, with the veins stretched on all sides as the animal the crab has its feet, whence it derives its name[95] (see picture). He later added the suffix -oma, Greek for swelling, giving the name carcinoma. Since it was against Greek tradition to open the body, Hippocrates only described and made drawings of outwardly visible tumors on the skin, nose, and breasts. Treatment was based on the humor theory of four bodily fluids (black and yellow bile, blood, and phlegm). According to the patient's humor, treatment consisted of diet, blood-letting, and/or laxatives. Through the centuries it was discovered that cancer could occur anywhere in the body, but humor-theory based treatment remained popular until the 19th century with the discovery of cells.
Our oldest description and surgical treatment of cancer was discovered in Egypt and dates back to approximately 1600 B.C. The Papyrus describes 8 cases of ulcers of the breast that were treated by cauterization, with a tool called "the fire drill." The writing says about the disease, "There is no treatment."[96]
Another very early surgical treatment for cancer was described in the 1020s by Avicenna (Ibn Sina) in The Canon of Medicine. He stated that the excision should be radical and that all diseased tissue should be removed, which included the use of amputation or the removal of veins running in the direction of the tumor. He also recommended the use of cauterization for the area being treated if necessary.[97]
In the 16th and 17th centuries, it became more acceptable for doctors to dissect bodies to discover the cause of death. The German professor Wilhelm Fabry believed that breast cancer was caused by a milk clot in a mammary duct. The Dutch professor Francois de la Boe Sylvius, a follower of Descartes, believed that all disease was the outcome of chemical processes, and that acidic lymph fluid was the cause of cancer. His contemporary Nicolaes Tulp believed that cancer was a poison that slowly spreads, and concluded that it was contagious.[98]
With the widespread use of the microscope in the 18th century, it was discovered that the 'cancer poison' spread from the primary tumor through the lymph nodes to other sites ("metastasis"). This view of the disease was first formulated by the English surgeon Campbell De Morgan between 1871 and 1874.[99] The use of surgery to treat cancer had poor results due to problems with hygiene. The renowned Scottish surgeon Alexander Monro saw only 2 breast tumor patients out of 60 surviving surgery for two years. In the 19th century, asepsis improved surgical hygiene and as the survival statistics went up, surgical removal of the tumor became the primary treatment for cancer. With the exception of William Coley who in the late 1800s felt that the rate of cure after surgery had been higher before asepsis (and who injected bacteria into tumors with mixed results), cancer treatment became dependent on the individual art of the surgeon at removing a tumor. During the same period, the idea that the body was made up of various tissues, that in turn were made up of millions of cells, laid rest the humor-theories about chemical imbalances in the body. The age of cellular pathology was born.
When Marie Curie and Pierre Curie discovered radiation at the end of the 19th century, they stumbled upon the first effective non-surgical cancer treatment. With radiation came also the first signs of multi-disciplinary approaches to cancer treatment. The surgeon was no longer operating in isolation, but worked together with hospital radiologists to help patients. The complications in communication this brought, along with the necessity of the patient's treatment in a hospital facility rather than at home, also created a parallel process of compiling patient data into hospital files, which in turn led to the first statistical patient studies.
Cancer patient treatment and studies were restricted to individual physicians' practices until World War II, when medical research centers discovered that there were large international differences in disease incidence. This insight drove national public health bodies to make it possible to compile health data across practises and hospitals, a process that many countries do today. The Japanese medical community observed that the bone marrow of bomb victims in Hiroshima and Nagasaki was completely destroyed. They concluded that diseased bone marrow could also be destroyed with radiation, and this led to the discovery of bone marrow transplants for leukemia. Since WWII, trends in cancer treatment are to improve on a micro-level the existing treatment methods, standardize them, and globalize them as a way to find cures through epidemiology and international partnerships.
Research
Cancer research is the intense scientific effort to understand disease processes and discover possible therapies. The improved understanding of molecular biology and cellular biology due to cancer research has led to a number of new, effective treatments for cancer since President Nixon declared "War on Cancer" in 1971. Since 1971 the United States has invested over $200 billion on cancer research, that total includes money invested by public and private sectors and foundations.
Label: Knowledge
Quotes...
Irasshaimase...
Search For Book...
Blog Archive...
-
▼
2008
(34)
-
▼
November
(26)
- Bingung???!!!
- Nostalgila....
- How To Editing My Blog...
- Hey!Say!JUMP
- Atashi no kareshii....
- How To Editing My Blog...(2)
- Lyrics....
- Semangat!!!
- Lyrics.... (2)
- Nobel Prize...
- Lyrics.... (3)
- The Art of War...
- Sad...
- Pencerahan.....
- Bingung???!!!
- Finally...
- HSJ + Ya-Ya-yah...
- Ganbare desu!!!
- Lyrics.... (4)
- Cancer Disease...
- Albert Einstein.....
- Ebola Disease...
- Biology...
- Biology... (2)
- DNA And Chtomosome...
- Atashi wa....
-
▼
November
(26)